UK NHS, Boehringer Ingelheim Taiwan undertake Coreline Comfortable imaging AI
South Korean imaging AI developer Coreline Comfortable has just lately bagged a couple of provide contracts in the UK, Europe, and Taiwan.
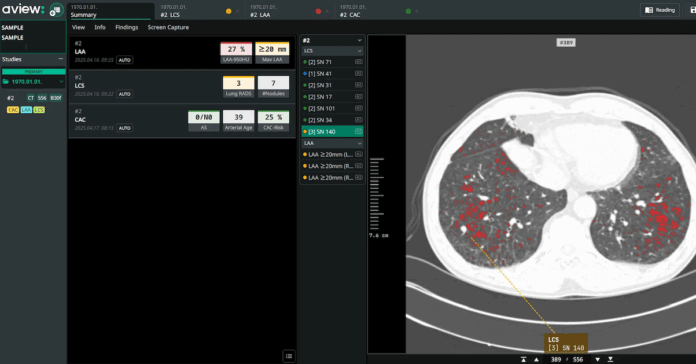
It has bought its first contract with the United Kingdom’s Nationwide Well being Provider (NHS) to supply its AI-powered scientific symbol research device AVIEW for the Japanese Diagnostic Imaging Community challenge, a collaboration of NHS Trusts in East England. The device shall be deployed to twelve hospitals, together with Cambridge College Health facility, for well being screening and basic CT examinations.
The corporate used to be additionally named the unique supplier of an AI symbol research device for the nationwide lung most cancers screening challenge of the French Nationwide Most cancers Institute (Institut Nationwide du Most cancers). Host hospitals, Help Publique – Hôpitaux de Paris and Lyon Town Health facility, selected Coreline Comfortable’s AI-powered lung most cancers screening resolution, which can discover pulmonary nodules, emphysema, and coronary artery calcification in one chest CT scan.
Additionally, Coreline Comfortable will ship its AI device module for figuring out lung deformation patterns to spouse hospitals of Boehringer Ingelheim Taiwan as a part of a program to check and test anti-fibrotic medication. The pharmaceutical corporate will use the lung development research module, which routinely hyperlinks chest CT research stories to PACS and RIS in close to actual time, to temporarily establish eligible sufferers with interstitial lung illness and abnormalities for medical trials of Ofev (nintedanib) and the investigational drug nerandomilast.
VUNO receives EU MD, UKCA marks
Fellow Korean scientific AI corporate, VUNO, has bought the Eu Union’s Clinical Tool Law certification and the United Kingdom Conformity Assessed mark for its flagship AI-powered cardiac arrest possibility review instrument.
In a commentary, the corporate stated those new regulatory clearances will permit it to arrange for equivalent certifications within the Center East, aiming to finish registration inside the yr and start supplying its device throughout hospitals within the area by way of subsequent yr.
VUNO’s cardiac arrest possibility review device, which is lately utilized in over 130 hospitals in South Korea, has pending clearance from the US Meals and Drug Management. In 2023, it used to be given a step forward software designation by way of the similar regulator.
Hong Kong OKs AITRICS’s affected person deterioration prediction AI
Any other scientific AI corporate from South Korea, AITRICS, additionally introduced its scientific software approval from the Hong Kong govt.
It has won the Hong Kong Clinical Tool Department’s clearance for its AI device, which analyses EMR knowledge to are expecting sufferers’ possibility of main antagonistic occasions, together with sepsis, cardiac arrest, ICU switch, and loss of life, inside of 4 to 6 hours.
That is its fourth regulatory approval following clearances from South Korea, the US, and Vietnam.
510(okay) for paediatric use of sensible wearable stethoscope
Singaporean corporate Aevice Well being has bought the United States FDA’s 510(okay) clearance for the expanded use of its sensible wearable stethoscope for far off breathing tracking in kids elderly 3 years and above.
The scientific software maker won its first 510(okay) for its AI-powered resolution in 2023.
Early this yr, it won overdue seed funding from chemical producer Denka via Pegasus Tech Ventures and undertaking capital company Elev8. The corporate has raised a complete of $9.1 million in reported investments since 2021.